STELARA® delivers rapid efficacy in UC
IN UC, STELARA® DELIVERS... EFFICACY FROM A SINGLE IV DOSE
1 dose of STELARA® reduced the symptoms† of UC as early as Week 22

† Week 2, the earliest scheduled study visit, and at each visit thereafter during UNIFI induction study.
‡ Symptomatic remission is defined as a Mayo stool frequency sub-score of 0 or 1 and a rectal bleeding sub-score of 0.
~87% of biologic non-failure patients achieved clinical response with STELARA® at either Week 8 or 163
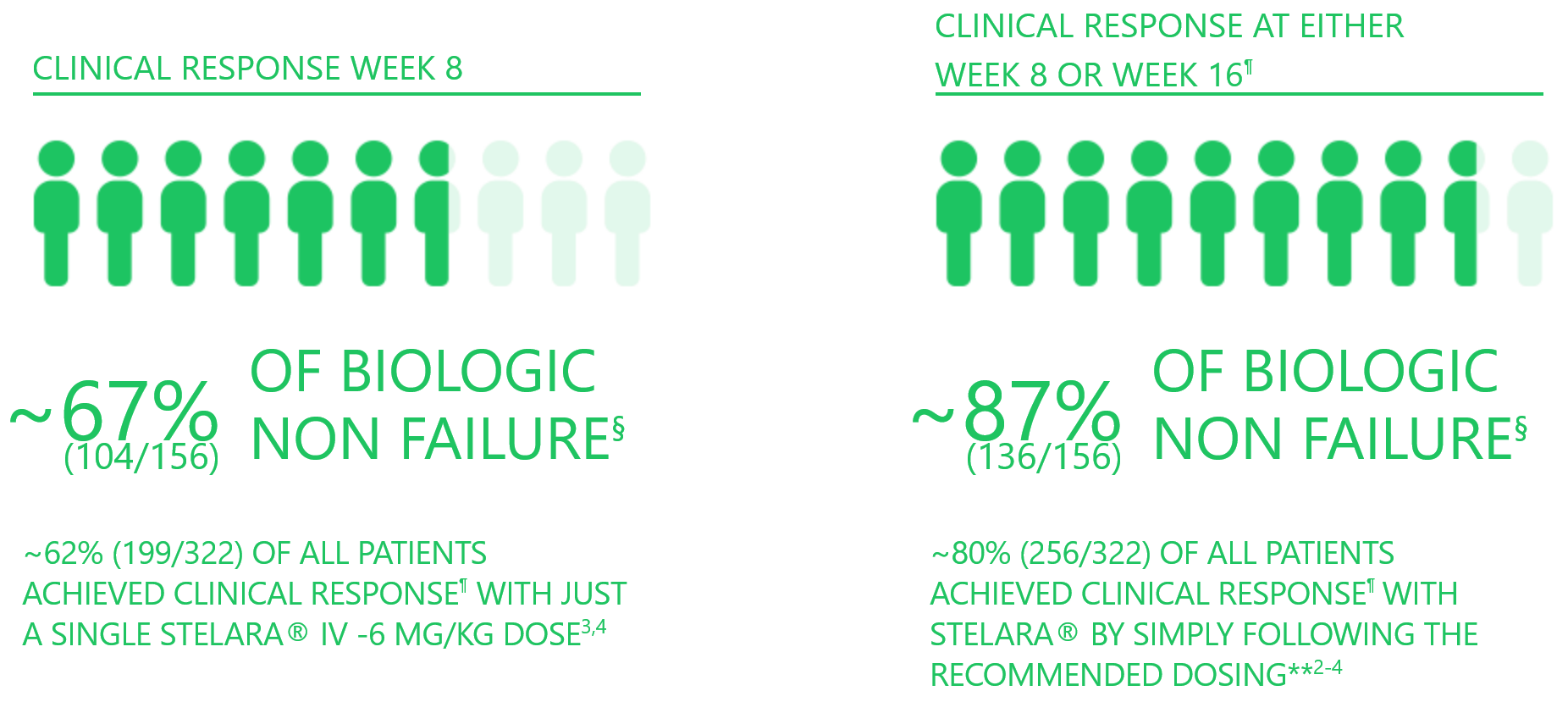
§ 93.4% biologic naive.
¶ Decrease from induction baseline in Mayo score (stool frequency, rectal bleeding, finding on flexible proctosigmoidoscopy and physician's global assessment) by >30% and >3 points, with either a decrease from induction baseline in rectal bleeding sub-score of 0 or 1.
** Clinical response at either Week 8 or Week 16. Patients not in clinical response at Week 8 received STELARA® 90 mg SC at Week 8 (as per recommended product dosing} and were re-analysed for clinical response at Week 16. Patients were counted only once if they achieved response at Weeks 8 and 16.
STELARA® in ulcerative colitis
Watch Prof. Elena Ricard talk about rapid UC efficacy in STELARA®
Efficacy from a single IV dose & high percentage of responders (UEGW)
Listen to Prof. Elena Ricart present an overview of the UNIFI induction data from STELARA® in UC.
* Long-term remission is considered a target of ideal treatment in the clinical management of ulcerative colitis (UC).1
IV: Intravenous; UC: Ulcerative colitis; SC: Subcutaneous.
References
- Danese S et al. Dig Dis 2019;37:266-283.
- STELARA® 130 mg concentrate solution for infusion. 90 mg / 45 mg solution for injection. Summary of Product Characteristics. February 2020.
- Danese S, et al. DOP54 presented at European Crohn’s and Colitis Organisation (ECCO) 14th Congress, 6–9 March 2019, Copenhagen, Denmark.
- Danese S et al. Poster Tu1739 presented at Digestive Disease Week (DDW) Annual Meeting, 18–21 May 2019, San Diego, CA, USA.
PRESCRIBING INFORMATION ADVERSE EVENTS REPORTING