STELARA® delivers lasting efficacy and minimal corticosteroid use in UC
IN THE UNIFI-LTE, STELARA® SUSTAINED SYMPTOMATIC REMISSION THROUGH 2 YEARS2
SYMPTOMATIC REMISSION OVER TIME THROUGH WEEK 92 FOR ALL PATIENTS WHO WERE RANDOMISED IN THE MAINTENANCE STUDY†3
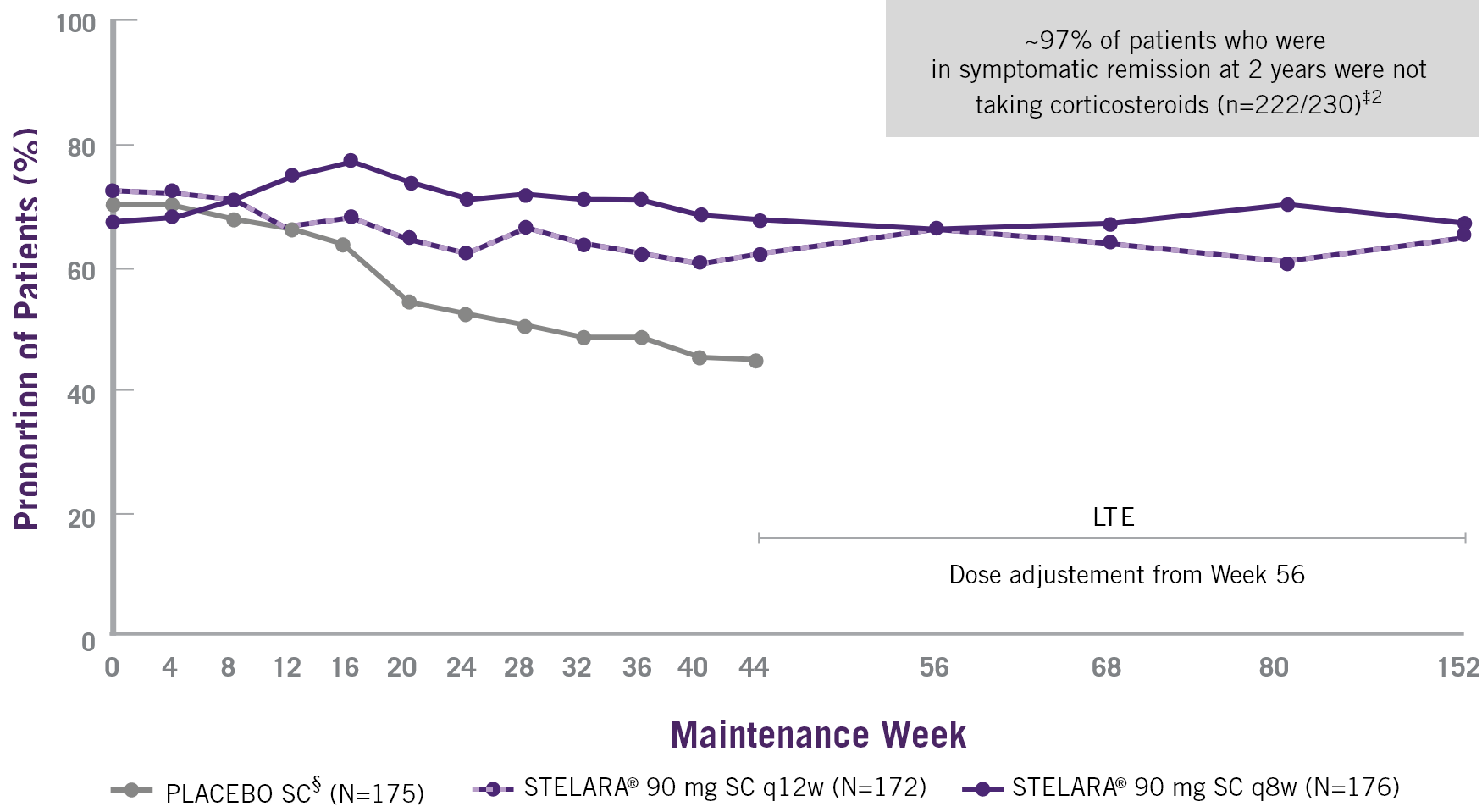
* Long-term remission is considered a target of ideal treatment in the clinical management of ulcerative colitis (UC).1
† Patients who were in clinical response 8 weeks after receiving a single STELARA® IV induction were randomised to placebo SC, STELARA® 90 mg SC q12w or STELARA® 90 mg q8w. All patients who completed Week 44 were eligible to enter and continue in the LTE at the investigator’s discretion.
‡ ITT analysis, dose-adjustment not considered a treatment failure.
§ Subsequent to completion of the maintenance study at Week 44, unblinding occurred and patients who were receiving placebo SC discontinued from the LTE.
STELARA® is now approved in ulcerative colitis
Watch Prof. Raja Atreya talk about lasting efficacy in STELARA®
Lasting Efficacy with limited use of CS
Listen to Prof. Raja Atreya present an overview of the UNIFI maintenance data of STELARA® in UC and the meaning of these results for patient care.
The importance of durable remission in UC
Watch Dr. Catherine Reenaers talk about durable remission in UC
The importance of durable remission in UC (ECCO)
Listen to Dr. Catherine Reenaers talk about the importance of durable remission in UC
IN UC, STELARA® ALLOWS FOR MINIMAL CORTICOSTEROID USE4
CLINICAL REMISSION AT 44 WEEKS (52 WEEKS AFTER STELARA® IV INDUCTION)
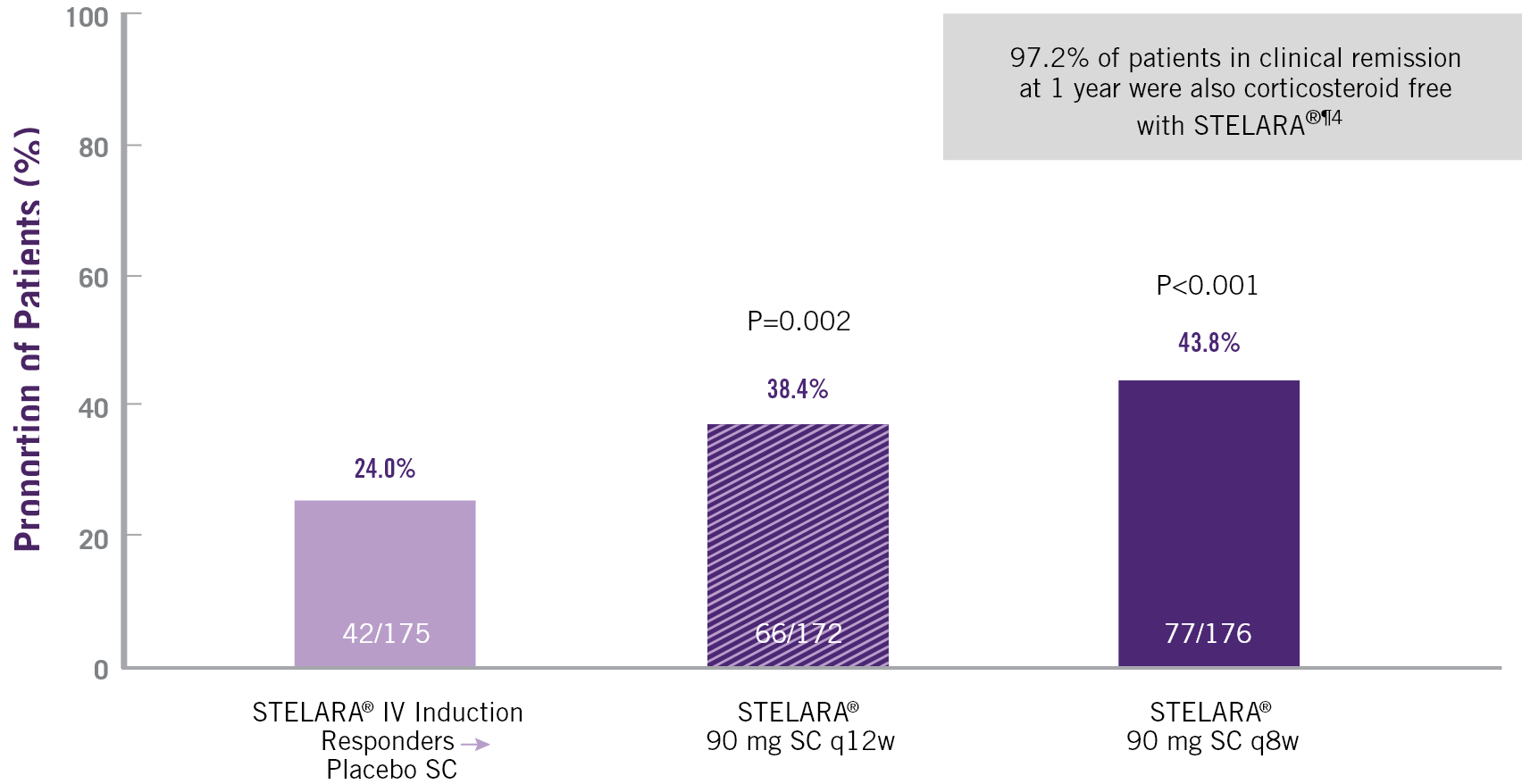
Clinical remission: Mayo score ≤ points with no individual sub-score >1. Corticosteroid-free remission: Patients in clinical remission and not receiving corticosteroids at Week 44 among those receiving concomitant corticosteroids at maintenance study baseline.
¶ Patients who were in clinical response with STELARA® IV induction dosing were randomised to placebo SC on entry into this maintenance study.
** Patients who were in clinical response 8 weeks after receiving a single STELARA® IV induction were eligible for the 44-week SC maintenance study (representing at least 52 weeks of treatment with STELARA®) and were randomised in a 1:1:1 ratio to placebo SC, STELARA® 90 mg SC q12w or STELARA® 90 mg q8w. This includes patients who responded to 130 mg IV induction.
The importance of durable remission in UC
Watch Prof. Andrea Pace
The importance of CORTICOSTEROID-FREE REMISSION in UC (ECCO)
Listen to Prof. Andrea Pace talk about the importance of corticosteroid-free remission in UC
HISTO-ENDOSCOPIC MUCOSAL HEALING
STELARA® is the first approved UC treatment to include in Phase III clinical trials (UNIFI induction and maintenance) the combined endpoint of histo-endoscopic mucosal healing5
In UC, histologic healing is associated with better clinical outcomes:6
- Lower rates of disease relapse
- Reduced risk of cancer
- Reduction in need for surgery/hospitalisation
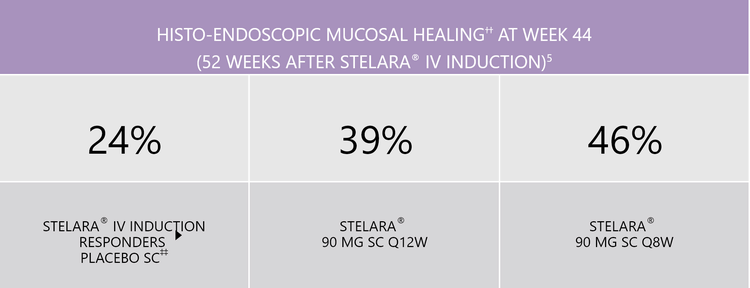
†† Histo-endoscopic mucosal healing was defined as Mayo endoscopy score of 0 or 1 with neutrophil infiltration in <5% of crypts and on crypt destruction, erosion, ulcerations or granulation tissue.
‡‡ Patients who were in clinical response with STELARA® IV induction dosing were randomised to placebo SC on entry into this maintenance study.
The importance of durable remission in UC
Watch Prof. Peter Irving
STELARA® in UC - Histo-endoscopic mucosal healing (UEGW)
Listen to Prof. Peter Irving present an overview of the histo-endoscopic mucosal healing data of STELARA® from the UNIFI study.
WATCH A PANEL OF EXPERTS DISCUSS THE IMPORTANCE OF MUCOSAL HEALING AS AN ENDPOINT IN UNIFI
* Long-term remission is considered a target of ideal treatment in the clinical management of ulcerative colitis (UC).1
CS: Corticosteroid; IV: Intravenous; UC: Ulcerative colitis; SC: Subcutaneous.
References
- Danese S et al. Dig Dis 2019;37:266-283.
- Panaccione R et al. Aliment Pharmacol Ther. 2020;52:1658-1675.
- STELARA® 130 mg concentrate solution for infusion. 90 mg / 45 mg solution for injection. Summary of Product Characteristics. February 2020.
- Peyrin-Biroulet L, et al. Clin Gastroenterol Hepatol 2014;12:929-934.
PRESCRIBING INFORMATION ADVERSE EVENTS REPORTING