STELARA® delivers consistent safety in UC
THE CONFIDENCE OF UP TO 11 YEARS’ HERITAGE IN 5 IMMUNE-MEDIATED INFLAMMATORY DISEASE INDICATIONS2,3
Combined data from 12 registrational trials in patients with psoriasis, psoriatic arthritis, and Crohn’s disease showed discontinuations due to adverse events (AEs) and incidences of AEs, serious AEs, and infections were consistent between STELARA® - and placebo-treated patients up to 1 year of follow-up.
IN UC, STELARA® HAS A SAFETY PROFILE COMPARABLE WITH PLACEBO AT 1 YEAR4
KEY SAFETY FINDINGS THROUGH WEEK 44 (52 WEEKS AFTER STELARA® IV INDUCTION)†4
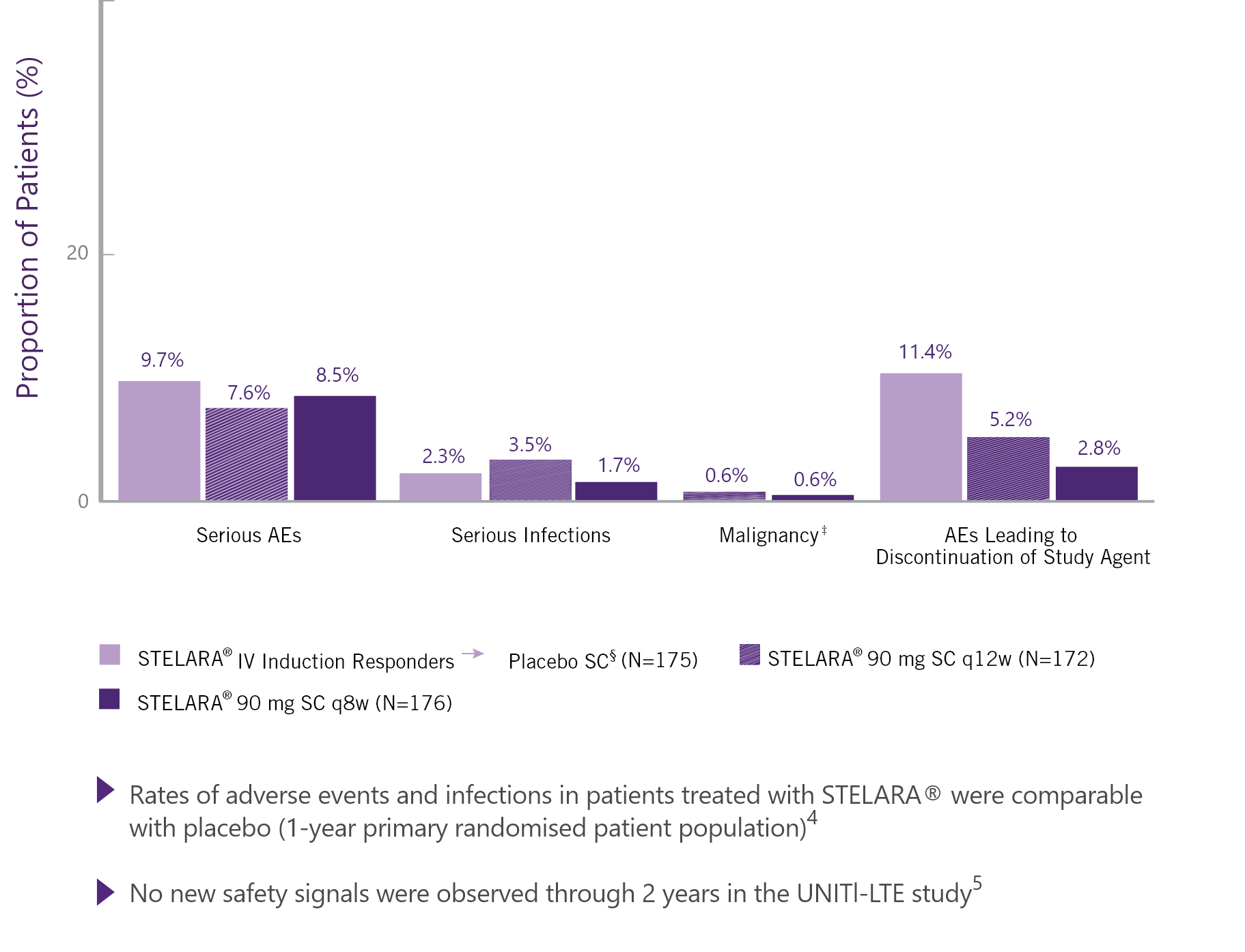
Data shown are for the UNIFI maintenance period only. Patients who were in clinical response 8 weeks after receiving a single STELARA® IV induction dose were eligible for the 44-week SC maintenance study.
‡ Papillary renal cell carcinoma in a patient from the STELARA® 90 mg SC ql2w group and colon cancer in a patient from the STELARA® 90 mg SC q8w group.
§ Patients who were in clinical response with STELARA® IV induction dosing were randomised to placebo SC on entry into this maintenance study.
STELARA® IS NOW APPROVED IN UC
Watch Dr. Krizstina Gecse talk about the safety data on STELARA®
Peace of mind through a consistent safety profile (UEGW)
Listen to Dr. Krisztina Gecse present an overview of the UNIFI safety data of STELARA® and its potential in UC treatment.
* Long-term remission is considered a target of ideal treatment in the clinical management of ulcerative colitis (UC).1
CS: Corticosteroid; IV: Intravenous; UC: Ulcerative colitis; SC: Subcutaneous.
References
- Danese S et al. Dig Dis 2019;37:266-283.
- STELARA® 130 mg concentrate solution for infusion. 90 mg / 45 mg solution for injection. Summary of Product Characteristics. February 2020.
- Ghosh S, et al. Drug Saf 2019;42(6):751-768.
- Panaccione R et al. Aliment Pharmacol Ther. 2020;52:1658-1675.
PRESCRIBING INFORMATION ADVERSE EVENTS REPORTING